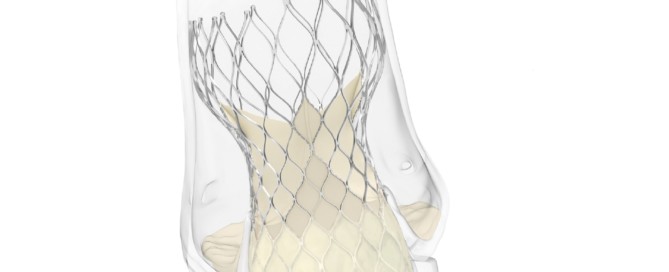
Evolut now has CE mark for low-risk patients and has new labelling for bicuspid use
Evolut Medtronic has been awarded CE mark for the use of the Evolut transcatheter aortic valve implantation (TAVI) system for patients with severe native aortic stenosis who are at low surgical risk. The Evolut TAVI platform has also received a new indication approval for the treatment of patients with bicuspid aortic valves [...]