Revivent TC
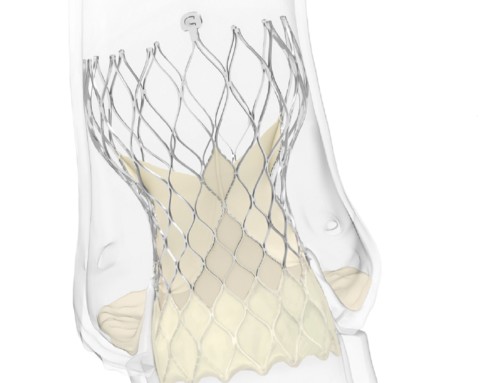
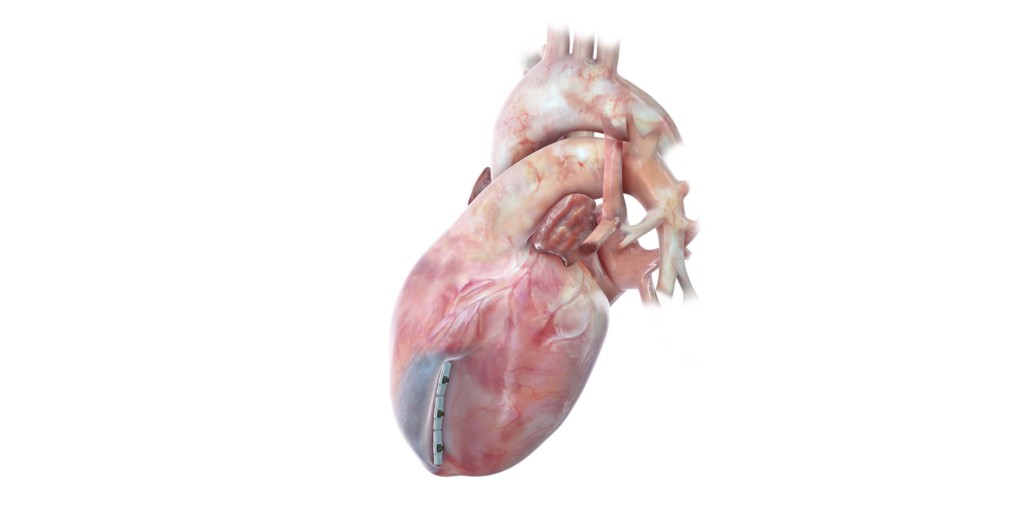
The FDA has granted Breakthrough Device Designation status for its Revivent TC transcatheter ventricular enhancement system (BioVentrix) for heart failure. A press release reports that the less invasive ventricular enhancement (LIVE) procedures uses Revivent TC System to exclude scar tissue on the left ventricle that has resulted from a myocardial infarction so the healthy portion of the heart can function more efficiently. It adds that the microanchors are implanted and designed to remodel the heart to a more normal shape and size and reduce wall stress, which has the potential to improve blood flow throughout the body.
The US pivotal ALIVE trial of the Revivent TC system is currently enrolling up to 120 patients at up to 20 US sites with a primary endpoint analysis at one year. The system has received the CE mark and is commercially available in Europe.
Andrew Wechsler (Drexel University College of Medicine, Philadelphia, USA), principal investigator for the ALIVE US investigational device exemption (IDE) trial of the Revivent TC system, says: “Heart failure continues to be an epidemic and the BioVentrix technology addresses a potentially curable cause of heart failure, which is precipitated by scarring of the left ventricle from a prior heart attack. Current methods for surgical remodelling of the ventricle are effective, but highly invasive, and not well tolerated by patients. Having a less invasive method to treat the ventricle enables more patients to benefit from a more efficient heart.”
BioVentrix CEO Ken Miller comments: “The breakthrough designation for the Revivent TC system recognises that many patients suffering from heart failure are at risk of death without a less invasive way to address their left ventricular damage. Being part of the Breakthrough Devices programme should help to speed the FDA’s evaluation and ultimately, market entry, so more patients can be helped more quickly.”