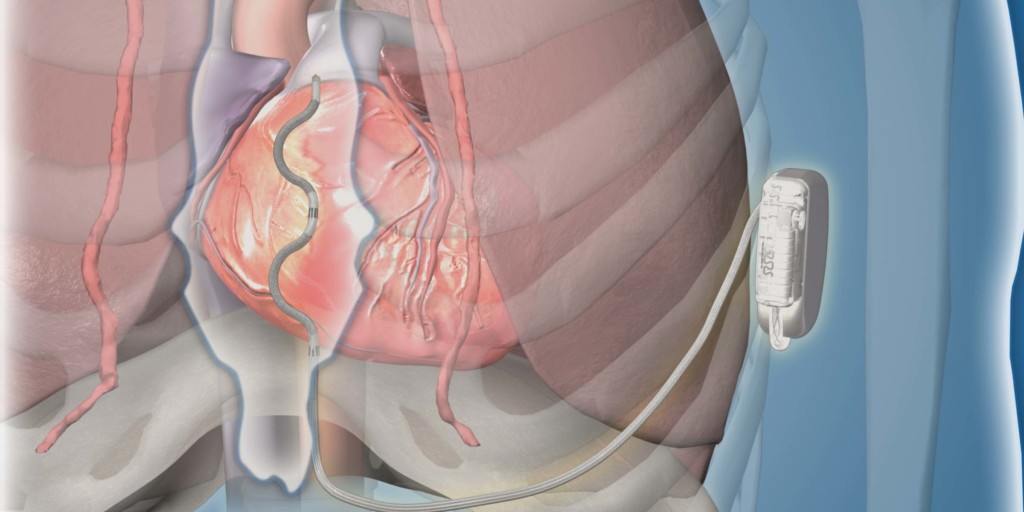
EV ICD
Medtronic has commenced a worldwide pivotal study to evaluate an investigational extravascular implantable cardioverter defibrillator (EV ICD) system for the management of tachycardia. A press release reports that the system is designed to provided defibrillation, as with a traditional ICD, but has a lead outside of the heart and veins.
It adds that the EV ICD device is implanted below the left armpit (in the left mid-axillary region), and that the lead is placed under the sternum. New procedure tools guide the delivery of the system. Furthermore, the novel device is the same size (33 cc) and shape as traditional (transvenous) ICDs, and is expected to have similar longevity.
The EV ICD pivotal study is a prospective, multicentre, single-arm, non-randomised, pre-market clinical study that will assess the EV ICD system in up to 400 patients at up to 60 sites in North America, Europe, the Middle East, Asia, Australia and New Zealand. The study’s primary effectiveness endpoint is defibrillation testing success rate at implant, and the primary safety objective is freedom from major system and/or procedural complications at six months after implant. Patients will be assessed at two weeks, three months, six months, and every six months thereafter.
Ian Crozier (Christchurch Hospital, Christchurch, New Zealand) and John Scherschel (Prairie Heart Institute, HSHS St John’s Hospital, Springfield, USA) performed the first implant in the study. The principal investigator for the study is Paul Friedman (Midwest Department of Cardiovascular Medicine, Mayo Clinic, Rochester, USA). Bradley P Knight (Center for Heart Rhythm Disorders at Northwestern Medicine’s Bluhm Cardiovascular Institute, Northwestern Memorial Hospital, Chicago, USA), another study investigator, comments: “Along with the other study investigators, I am enthusiastic to evaluate the safety and efficacy of this new approach, and its potential to deliver lifesaving ICD therapy without the risks associated with leads inside the veins and heart, and without compromising on the features available in traditional ICDs.”
Rob Kowal (chief medical officer, vice president of medical affairs in the Cardiac Rhythm and Heart Failure division, which is part of the Cardiac and Vascular Group at Medtronic) says: “With the EV ICD system, we aspire to deliver a new standard in ICD therapy for patients who are at significant risk of life-threatening rhythms in the lower chambers of the heart. Our engineers have spent years developing this innovative system, and we appreciate the high level of interest and collaboration among our expert investigators and regulatory authorities worldwide to carry out the EV ICD pivotal study.”
The EV ICD pivotal study follows the successful first-in-human chronic EV ICD pilot study, which evaluated 21 patients in New Zealand and Australia. The results of this pilot study were presented as a late breaking clinical trial at Heart Rhythm 2019 (8–11 May, San Francisco, USA).





