Click edit button to change this text.
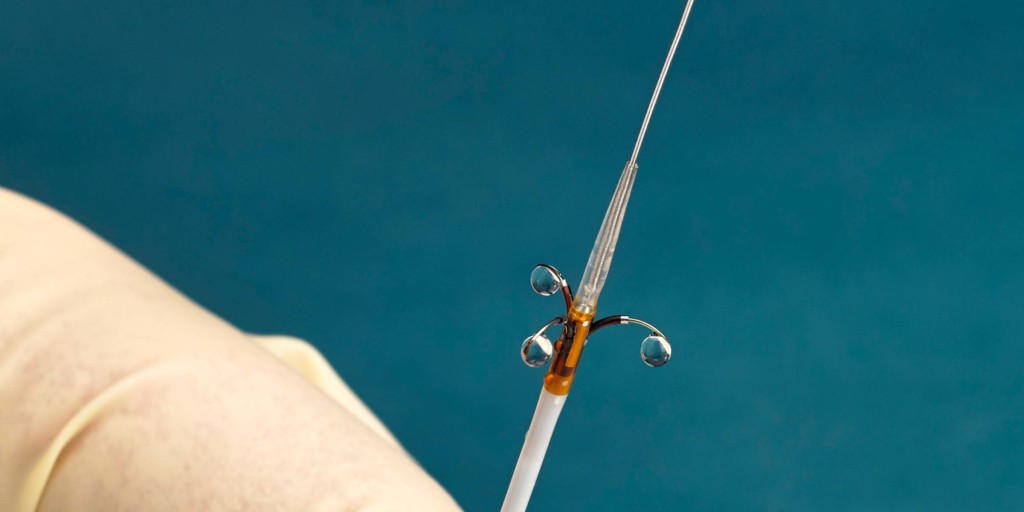
Peregrine system kit
Ablative Solutions has announced the enrolment and randomisation of the first patient in its TARGET BP I global clinical trial, which is evaluating Peregrine system kit for the treatment of patients with uncontrolled hypertension. The Peregrine system kit, which is comprised of a patented infusion catheter and dehydrated alcohol, is used in a minimally invasive procedure with the goal of deactivating the nerves surrounding the renal arteries and thereby reducing blood pressure.
Both the global TARGET BP I trial and the ongoing European TARGET-BP OFF-MED trial are part of the TARGET BP clinical trials programme and are designed to evaluate the safety and efficacy of the Peregrine system for patients with uncontrolled hypertension. Piedmont Heart Institute (Atlanta, USA) was the first hospital centre worldwide to enrol and randomise a patient into the TARGET BP I clinical trial. David Kandzari (Piedmont Heart Institute, Atlanta, USA)—co-principal investigator of the TARGET BP I trial—says: “It is exciting to initiate this important trial to evaluate the Peregrine system kit for the treatment of patients with uncontrolled hypertension. More than half of those treated with antihypertensive medications do not achieve their target blood pressure, so there is a great need for improved therapeutic options.”
Data from the TARGET BP I trial will be used to support regulatory approval in the USA for the product’s use for the treatment of hypertension, in conjunction with medical therapy. Data will also support the European approval of the medicinal product for the treatment of hypertension.
Tim Fischell, chief medical officer at Ablative Solutions, comments: “A European postmarket study assessing a similar combination, which used the CE-marked Peregrine catheter with alcohol as a neurolytic agent, provided promising early data that supported moving into the pivotal clinical studies. That study showed a reduction in mean systolic 24-hour ambulatory blood pressure of 11mmHg (±14 mmHg, p<0.001) at six-month follow-up, and demonstrated a strong signal of safety.”





