Helix
BioCardia has announced the renewal of the CE mark for its Helix biotherapeutic delivery catheter and Morph universal deflectable guide catheter—both are used in the delivery of biotherapeutics to the heart. A press release reports that the CE mark renewal enables BioCardia to continue supporting partners conducting biotherapeutic clinical trials outside of the USA, such as CellProThera and Centro Cardiologico Monzino, as well as commercial sales in the European Union and other countries that recognise the CE Mark through May 2024.
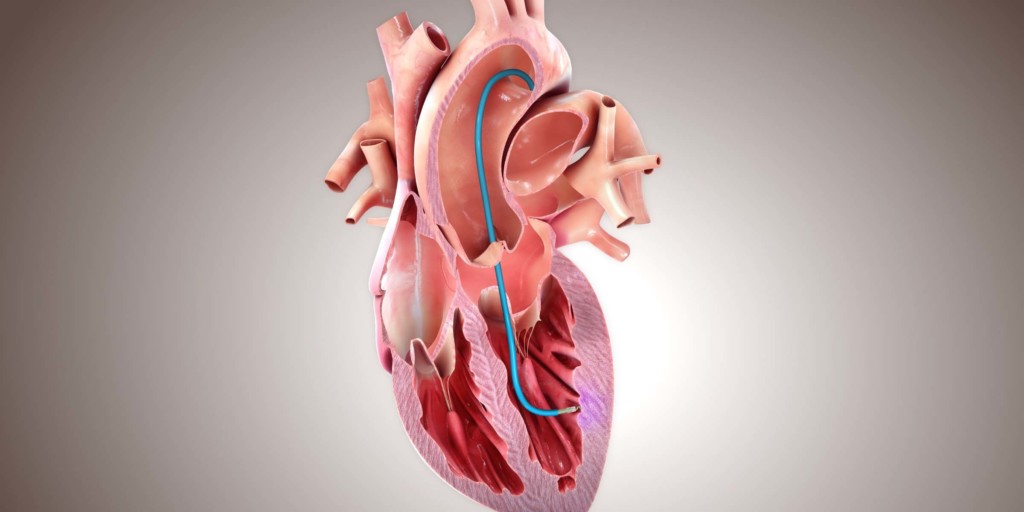
The press release adds that published literature has shown that the Helix biotherapeutic delivery system, which includes the Morph guide product, presents the lowest risk to patients for biotherapeutic delivery compared to other delivery methods evaluated. Furthermore, according to these data, the system is three to six times more efficient at delivering cells to the heart muscle than other methods. The Helix system is currently being evaluated in a USA phase III pivotal CardiAMP heart failure trial along with the company’s lead cell therapy candidate (CardiAMP), which uses a patient’s own (autologous) bone marrow cells delivered to the heart in a minimally-invasive procedure to potentially stimulate the body’s natural healing response.
BioCardia CEO Peter Altman says: “Renewal of the CE mark for our innovative biotherapeutic delivery products ensures that we can continue participating in a variety of biotherapeutic research programs with the potential to drive the field forward and respond to very ill patients. In light of sweeping European regulatory changes associated with the transition from the medical device directive (MDD) to the new medical devices regulation (MDR), it has been a challenging time for both notified bodies, who are working to become certified to grant CE mark under the new system, and for companies, who are trying to meet the new requirements and move their CE Mark applications forward. It is a significant achievement for BioCardia to have received CE Mark renewal in this environment, when many other companies are struggling to do the same or even abandoning the region.”