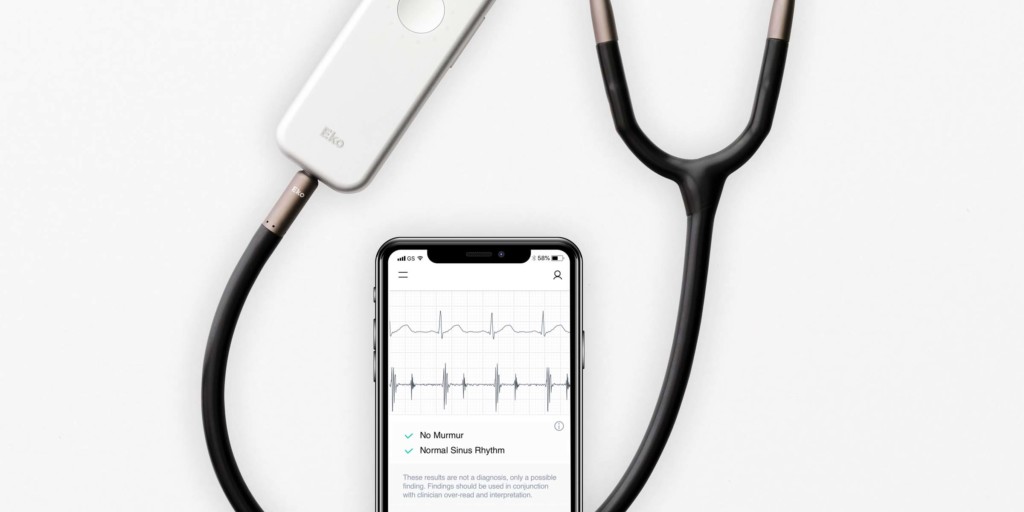
A press release reports that the FDA has cleared a suite of algorithms that, when combined with Eko’s digital stethoscopes, will enable healthcare providers in the USA to more accurately screen for heart conditions during routine physical exams. If left undiagnosed, these heart conditions can lead to stroke and heart failure.
According to the press release, Eko’s artificial intelligence (AI) is able to identify heart murmurs; a recent study revealed that using traditional stethoscopes, primary care physicians had a sensitivity of 43% and a specificity of 69% for detecting significant valvular heart disease. The AI is able to detect atrial fibrillation with 99% sensitivity and 97% specificity when analysing the one-lead ECG tracing from the Eko DUO stethoscope. The integration of ECG into the stethoscope enables providers to quickly screen patients for the serious arrhythmia during a standard physical exam.
The Algorithm also reports heart rate and QRS duration and identifies tachycardia and bradycardia, abnormally fast and slow heart rates, which can be indicative of heart disease or other health conditions such as thyroid disease.
Connor Landgraf, Eko’s co-founder and CEO, comments: “Our vision since day one has been to build seamless technology that helps providers more accurately detect heart disease, the leading killer in the world, by putting the ears of a cardiologist in any clinician’s stethoscope. Eko’s new ability to alert a provider to the presence of a heart murmur or atrial fibrillation during the standard physical exam brings that vision to life.”
In December 2019, Eko announced the FDA had granted the company breakthrough status for a novel ECG-based algorithm that, if FDA-cleared, could provide an easily accessible screening test for heart failure. Since the release of its first-generation Eko CORE device in 2015, Eko’s technology has been adopted by clinicians at more than 4,000 hospitals and clinics in the USA and Europe.





