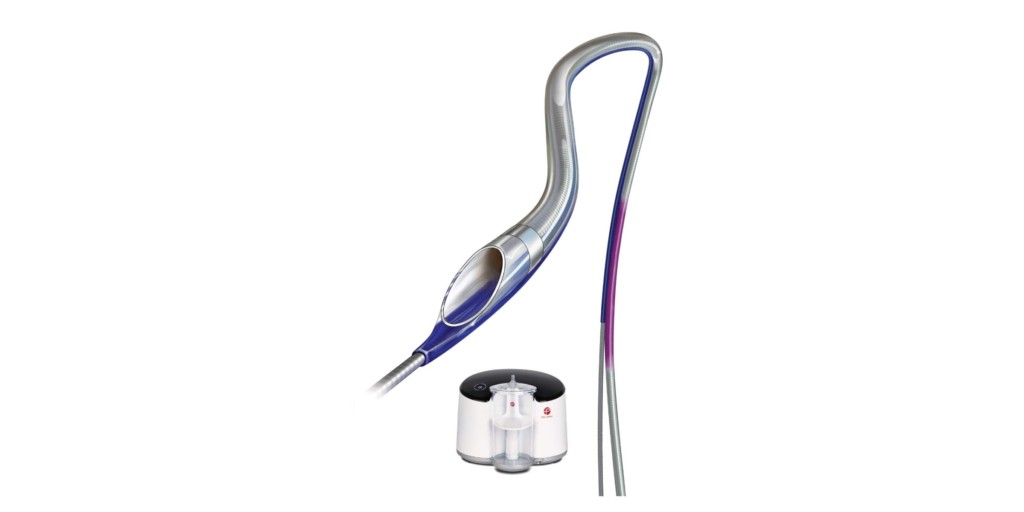
CAT RX
Penumbra has announced the enrolment of the first patient in its CHEETAH study, a prospective, multicentre US study to evaluate the safety and performance of the Indigo system with CAT RX aspiration catheter in the coronary vessels. Introduced in 2018, as part of the Indigo aspiration system, the Indigo CAT RX aspiration catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature.
The Indigo system CAT RX device uses mechanical power aspiration to remove thrombus in the coronaries. The postmarket, prospective CHEETAH study will enrol up to 400 patients presenting with coronary thrombus who are referred for percutaneous coronary intervention (PCI) at up to 25 US centres. The primary study endpoint is a composition of cardiovascular death, recurrent myocardial infarction, cardiogenic shock or new or worsening New York Heart Association (NYHA) Class IV heart failure within 30 days. Secondary endpoints include final TIMI flow grade, final TIMI thrombus grade, and safety assessments at six months.
S Jay Mathews (Manatee Memorial Hospital, Bradenton, USA), the national principal investigator, comments: “Penumbra has adapted over 10 years of neuro thrombectomy experience to address the limitations of traditional manual aspiration for the coronaries by development of the Indigo system CAT RX device. This significant upgrade in innovation from syringe-based aspiration to mechanical power aspiration coupled with highly trackable catheter technology has enabled us to improve our door to reperfusion times and thereby to improve patient care.”
Jasvindar Singh (Barnes Jewish Hospital/ Washington University School of Medicine, St Louis, USA) says: “Just like the IMS-III trial for ischaemic stroke, the TOTAL trial highlighted that new therapies are needed to improve the outcomes of these patients with high thrombus burden. The CHEETAH study is the first step toward making coronary mechanical thrombectomy standard of care for high thrombus burden patients. We expect this study to refine our technique of thrombus aspiration and better understand the utilisation of CAT RX for patients with high thrombus burden.”





