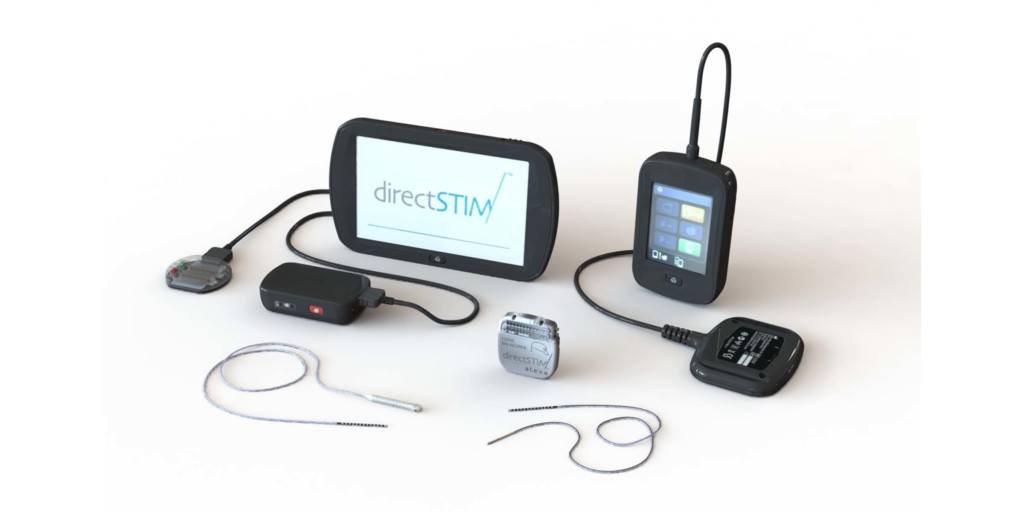
DirectSTIM
Aleva Neurotherapeutics has been awarded the CE mark for its—a press release reports—flagship product, the directSTIM deep brain stimulation (DBS) system. According to the press release, Aleva’s revolutionary DBS system incorporates directional electrode technology and is designed to be more precise and efficient, with optimised stimulation that will potentially reduce side effects. At present, Aleva is the only emerging technology company to be awarded the CE-mark for DBS.
The company will initiate direct sales through a post-market clinical follow-up study in select European neurological clinics. Furthermore, it has announced it has raised $8m in a first closing of a Series E financing round from existing and undisclosed new private investors. A limited additional amount of the round remains open to qualified investors.
With its European market entry secured, the press release states, the Aleva Neurotherapeutics will now drive its efforts towards US market entry. The company has been riding a wave of momentum driven by the uniqueness of its core technology and the major unmet needs in treating neurological disorders. Previously, the successful results of Aleva’s directSTN pilot study were published in Brain and strongly suggested the potential of directional stimulation in improving surgical outcomes. Earlier this year, Aleva announced the successful creation and funding of a joint venture with Dixi Medical SAS named Adept Neuro SA that aims to achieve regulatory approval for its core technology in the field of epilepsy surgery.
André Mercanzini, CEO of Aleva Neurotherapeutics, comments: “The CE mark is a significant milestone in Aleva´s development. Our DBS system incorporates first-in-class directional electrode technology with an easy-to-use patient programming interface that will enable functional neurosurgery teams to provide better outcomes for their patients. We have demonstrated that we can achieve and maintain technological leadership in our field, and we are now ready to expand our market in Europe while remaining focused on the US FDA regulatory process.”





