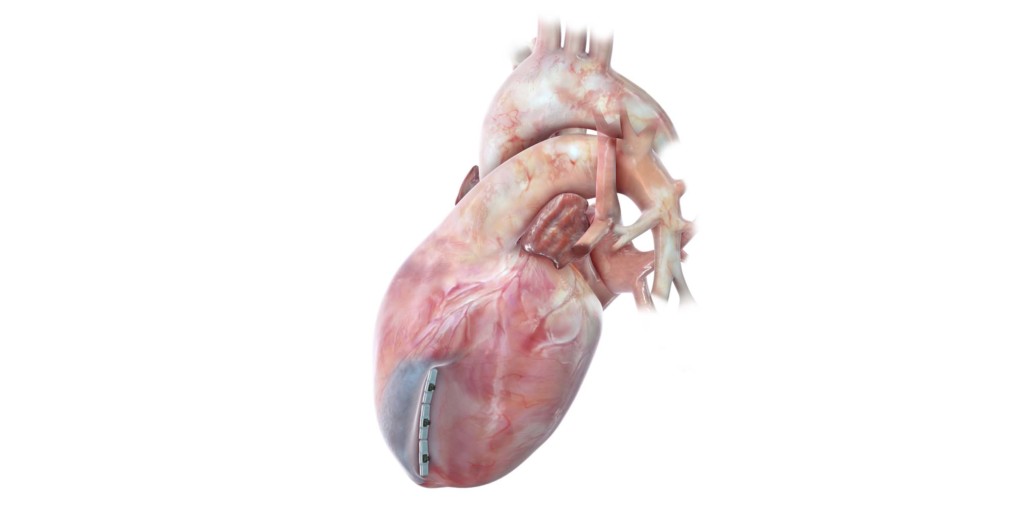
BioVentrix
BioVentrix has announced that Gregg Stone (Mount Sinai Health System, New York, USA) has signed on as principal investigator of the ALIVE trial of the Revivent TC transcatheter ventricular enhancement system. The system is a hybrid, closed-chest transcatheter procedure designed to treat patients with ischaemic cardiomyopathy by reshaping and restoring the left ventricle.
Stone has served as the national or international principal investigator for more than 100 national and international multicentre randomised trials, many of which have led to FDA clearance of new medical devices or indications in the USA. Additionally, he has authored more than 2,000 manuscripts and abstracts published in peer-reviewed literature, as well as numerous book chapters, and is a highly sought speaker at cardiology seminars around the world.
The ALIVE trial is designed to demonstrate the safety and effectiveness of the Revivent TC system. In the procedure, with the system, microanchors are implanted in the left ventricle to exclude scarred myocardium from the healthy tissue. The aim with the trial is to enrol 120 patients at up to 20 sites in the USA with a primary endpoint analysis at one year. The trial endpoints include positive effects on volume reduction, ejection fraction, quality of life, New York Heart Association (NYHA) failure class compared to baseline, exercise capacity, and rehospitalisation.
Stone comments: “The LIVE therapy using the Revivent TC system is one of the most promising approaches I have seen for the lesser invasive treatment of left ventricular scarring from ischaemic cardiomyopathy. I am excited about the potential for this therapy to improve prognosis and quality of life for suffering patients with advanced heart failure.”





