Appligator
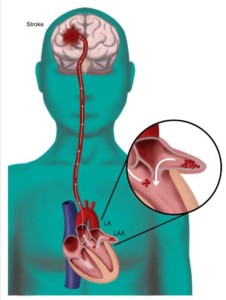
Append Medical has announced the clinical proof-of-concept data evaluating the safety of left atrial appendage (LAA) closure through Append’s patented tissue manipulation procedure. A press release says that the procedure serves as a feasibility study for Append’s Appligator minimally invasive LAA closure device, which uses natural tissue manipulation to achieve complete LAA closure through invagination of the LAA into the left atrium, leaving only a suture to seal the area.
The Appligator device is designed to reduce stroke risk in atrial fibrillation patients by completely closing the LAA to prevent blood clot leakage with a minimally invasive transseptal intervention. Its design is intended to minimise device-related thromboembolism risk by leaving only a suture—and no metal implant—at the closure site.
Leonid Sternik (Department of Cardiac Surgery, Sheba Medical Center, Ramat Gan, Israel), co-inventor of the Appligator device and director of the, performed the procedures in open heart surgery on two patients, both of whom have atrial fibrillation, in clinical safety trials. In follow-up after three years and one year respectively, each patient is healthy and the invaginated LAA has been successfully absorbed into the surrounding tissue of the left atrium. He says: “These clinical safety trials showcase the efficacy of tissue manipulation for LAA closure in atrial fibrillation patients. The LAA is a complex structure, which may lead to complications when using implanted devices for sealing. Using the LAA tissue itself for closure with no implanted device left behind can reduce risks of device-related thromboembolism and device embolisation. We believe this approach can significantly simplify LAA closure, making it safer and more effective for patients with AF-related stroke risk.”
Zachi Berger, founder and CEO of Append Medical, comments: “By developing a safer, more effective LAA closure procedure, Append is accelerating the shift from blood thinners to transseptal interventions for stroke risk reduction in AF patients, proving its value as a differentiated solution in the LAA closure market.”





