Source: BIBA MedTech Aortic Segmentation Monitor
Aortic Insights is a quarterly report that provides in-depth analysis of the key findings of the Aortic Segmentation Monitor (BIBA MedTech Insights). Each quarter, the monitor tracks aortic procedures performed in Western Europe. It looks at the segment treated (infrarenal, complex, and thoracic), type of procedure (endovascular, hybrid, and open), and devices used. In this edition of Aortic Insights, in Market Insights, we review the Q1 (1 January–31 March) results of 2019. We also look at the latest technology news (Technology Insights) and pipeline developments (Pipeline Insights).
Market Insights
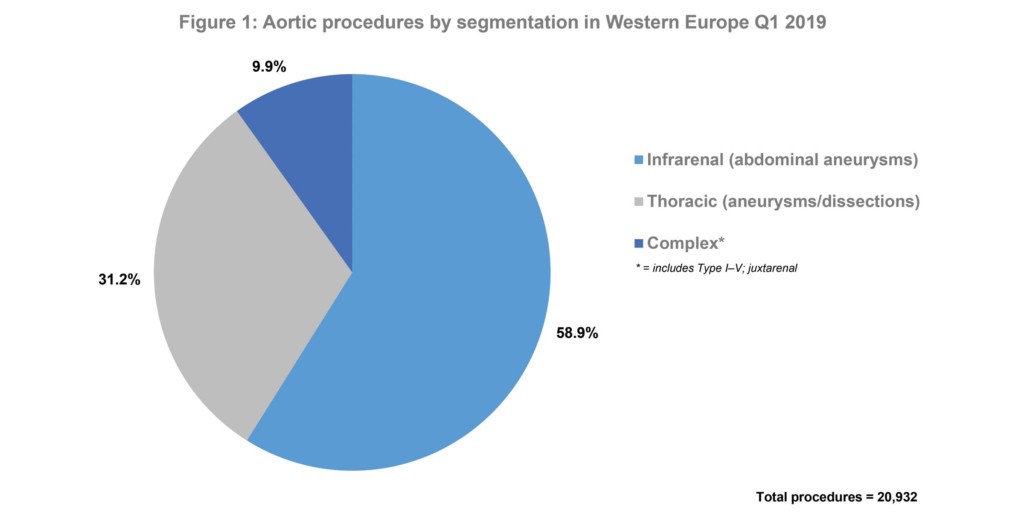
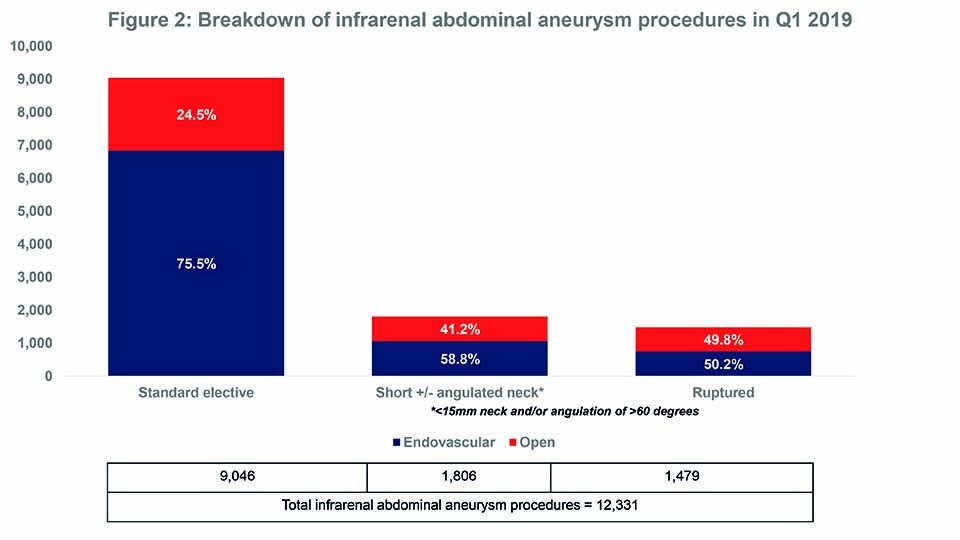
- Of 20,932 aortic procedures performed in Western Europe in Q1 2019, nearly 60% were for infrarenal abdominal aneurysms.
- The endovascular approach is, by far, the most common method of treating infrarenal abdominal aneurysms.
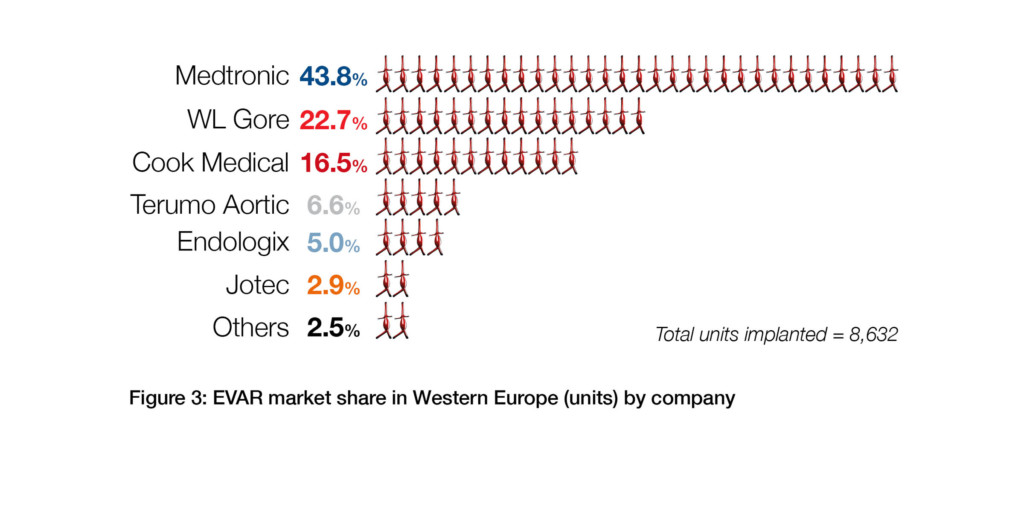
- Medtronic is the leader of the endovascular aneurysm repair (EVAR) market in Western Europe.
Source: BIBA MedTech Aortic Segmentation Monitor
In Q1 2019, 20,932 aortic procedures were performed in Western Europe. Of these, 58.9% were for infrarenal abdominal aneurysms. See Figures 1 and 2. Overall, the endovascular approach was, by far, the most common method of treatment. Additionally, during Q1 2019, 8,632 endovascular aneurysm repair (EVAR) devices were implanted. Of these, 43.8% were Medtronic’s Endurant II stent graft systems—making Medtronic the market leader. See Figure 3.
The Endurant II range is indicated for infrarenal abdominal aneurysms and for aortoiliac aneurysms. Medtronic reports that the systems can be used for patients with adequate iliac or femoral access; a proximal neck length of ≥10mm; infrarenal neck angulation of ≤60 degrees; aortic neck diameters of 19–32mm; distal fixation length of ≥15mm; iliac diameters of 8–25mm, and morphology suitable for aneurysm repair.1
Source: BIBA MedTech Aortic Segmentation Monitor
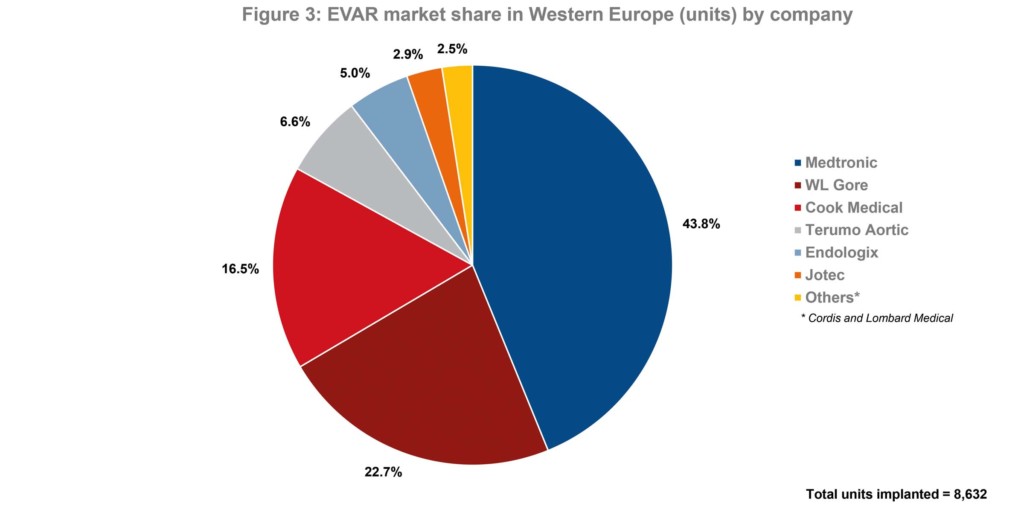
Source: BIBA MedTech Aortic Segmentation Monitor
EndoAnchors
The Endurant II bifurcated stent graft system can, however, also be used in patients with a proximal neck length of between ≥4mm and <10mm if used in conjunction with the Heli-FX EndoAnchor system. According to data from the monitor, 4% of all endovascular infrarenal aneurysm procedures performed in Q1 2019 (8,632) were with the Heli-FX EndoAnchor system (with an average of five EndoAnchors used per procedure). Furthermore, of 1,685 EndoAnchors implanted, 54.9% were for procedures in short and/or angulated necks vs. 45.1% for standard elective procedures.
At the 2019 Charing Cross Symposium (15–18 April, London, UK), Frank R Arko (Department of Vascular and Endovascular Surgery, Carolinas Medical Center, Charlotte, USA) presented the preliminary two-year results from the ANCHOR registry.2 Of 38 patients who underwent EVAR with the combined Endurant II/Heli-FX approach, for whom two-year follow-up data were available, freedom from aneurysm-related mortality was 94.3%. Additionally, freedom from a secondary procedure was 95.5%. Other findings show that no new or persistent type 1a endoleaks, according to core lab assessment, were identified; 64.7% of patients experienced aneurysm sac regression of >5mm, and no patients had evidence of sac enlargement. Arko commented: “By using an EndoSuture aneurysm repair (ESAR) approach with the Endurant stent graft and the Heli EndoAnchor system, physicians are able to safely and effectively treat a wider range of patients with short aortic neck anatomies, independent of renal stenting. At two years, the preliminary data also demonstrate that 100% of sacs are either shrinking or stable—an indicator of EVAR durability.”
Technology Insights
“Unprecedented” feedback leads to ongoing delays for NICE abdominal aortic aneurysm guidance
In May last year, the National Institute for Health and Care Excellence (NICE) published draft guidance for the diagnosis and management of abdominal aortic aneurysms. If implemented, the new recommendations could radically change the way that aneurysms are managed in England and Wales as they advise against the use of endovascular aneurysm repair (EVAR) for patients with unruptured aneurysms. However, because of the volume of concerns about the draft guidance, NICE are still in the process of reviewing their recommendations and have yet to finalise them.

Ian Loftus
The president of the Vascular Society of Great Britain and Ireland (VSGBI) Ian Loftus, in an interview with Vascular News, says that the amount of feedback to the proposed recommendations was “unprecedented” and this has prompted “quite an unusual process”.3 He says: “First, NICE delayed [finalising] the guidance and, secondly, they put it through an internal view. The VSGBI understands that this review has now been completed and further consultation took place within the guidance committee. Subsequent to that, there has been additional discussions among the various stakeholders. All of this has culminated in the presentation of the draft guidance to the NICE board. So, the current situation is that the guidance sits with the NICE board.” On their website, NICE acknowledge that the continued delay to the finalising of the guidance “has led to uncertainty amongst clinicians and service providers”.4 They add: “However, we want to ensure that all of the issues raised at consultation, including the management of abdominal aortic aneurysms, service delivery, and the need for patient information, are properly addressed, and the guideline has the confidence of not only the clinicians who will apply it, but more importantly, the patients whose treatment it will inform.”
The controversy surrounding the draft guidance, according to Loftus, relates to its recommendations barring the use of EVAR for all patients with unruptured aneurysms—regardless of their suitability for surgery and regardless of the complexity of the procedure. The guidance reports that the rationale for these recommendations is based on evidence (or lack thereof) for the long-term benefits of EVAR vs. surgery for unruptured aneurysms.4 It notes: “While EVAR is associated with fewer perioperative deaths, it has more long-term complications, and these complications mean that people will need further procedures.”
Given that EVAR is now the predominant approach used to treat infrarenal aneurysms in the UK, a shift back to surgery may have implications for the provision of vascular services. Loftus says there would be a “much greater need for resources” as more patients undergoing surgery would mean more patients staying in hospital for longer (and thus, needing beds for longer) and operating theatres being occupied for longer as surgical procedures “take longer”. Another issue is that there may not be enough vascular surgeons with adequate training to perform open surgery. “We now have a workforce that is largely deskilled in open surgery, especially complex aneurysm surgery. Therefore, a move towards surgery would have big implications for training existing consultants. There are also implications for vascular trainees who are coming through the system and who really want to be involved in modern endovascular technologies. If this draft guidance were implemented, I think we might find we lose quite a lot of training resources.”
The draft guidance, however, does not completely ban the use of EVAR as it actually recommends EVAR over surgery for the majority of patients with ruptured aneurysms. It says: “EVAR provides more benefit than open surgical repair for most people, especially for women and for men over the age of 70. Open surgical repair is likely to provide a better balance of benefits and harms in men under the age 70”. Loftus, though, believes that this preference for EVAR for ruptured aneurysms is also beset by difficulties. “Providing a service in an emergency setting that we do not provide in a routine setting? I think that is unworkable,” he explains.
Ongoing dialogue
Although the guidance is still to be finalised, there is not stalemate between the NICE committee and the interested stakeholders. Loftus comments: “We are unclear about the next step, but what we are clear about is that NICE are listening to us and our concerns. We remain in dialogue with them to find a workable solution to the NICE guidance”.
Additionally, according to Loftus, most clinicians “accept that perhaps we have taken things too far with endovascular technologies” and that a reappraisal of how EVAR is used is needed. He says: “I do think we need to consider whether endovascular technologies are inappropriate for elderly patients, for frail patients, or those with small aneurysms. Also we need to review whether surgery might be better for young patients with a very long life expectancy.” Ultimately, Loftus believes, a compromise is needed between the draft guidance’s “unacceptable” approach of not offering EVAR to elective patients and the current approach of using EVAR as the first-line approach for most patients.
What both NICE and the Loftus agree on is the need for more research. The draft guidance outlines priorities for research including the effectiveness of EVAR compared with surgery for both ruptured and unruptured aneurysms. Equally, Loftus says: “We need much better work on who is going to benefit the most, or who is going the least, from which approach. We also need better selection criteria so we can guide patients as to what we think is the best option for them.”
The implications of the NICE guidance, when finalised, may not just affect the UK. Loftus comments: “NICE guidelines are well quoted internationally and can have an impact on the provision of services outside of the UK. The processes by which guidelines are drawn up are complex and generally recognised as robust, in terms of the methodology and conclusions drawn. Therefore, other health care systems do sometimes take note of the recommendations made.”
Top findings
British Society of Endovascular Therapy 2019 Annual Meeting (BSET; 27–28 June, Wotton-under-Edge, UK): An interplay of anatomical and physiological factors in patients with complex aneurysms renders conventional risk stratification tools “unhelpful” as it is “unusual” that a single major factor ultimately determines treatment modality. Therefore, a multidisciplinary approach to assessment is mandated.5
BSET 2019: An endovascular approach is “safe and effective” for the management abdominal aortic aneurysms that have been detected by the National Abdominal Aortic Aneurysm Screening Programme (NAAASP). Furthermore, according to this cohort, there was no aneurysm-related mortality in those treated electively for infrarenal aneurysms and the overall reintervention rate was relatively low.6
Society of Vascular Surgery (SVS) Vascular Annual Meeting 2019(VAM 2019; 12–15 June, National Harbor, USA): Five-year data from the INSPIRATION US investigational device exception (IDE) pivotal trial confirms favourable safety and effectiveness of the INCRAFT device (Cordis). Michel Makaroun, University of Pittsburgh (Pennsylvania, USA) surmised that the INCRAFT device “offers unique benefits in patients with challenging access anatomy” while it “may provide a useful option for patients with infrarenal abdominal aortic aneurysms”.7
Critical Issues 2019 (23–24 May, Liverpool, UK): A single-centre study of standard, fenestrated and branched endovascular aneurysm repair patients in Nürnberg, Germany, has found that outcomes for women are worse but the reasons are not yet clear, as literature on the topic is lacking.8
Charing Cross Symposium 2019 (CX 2019; 15–18 April, London, UK): Using EndoAnchors (Medtronic) to fix and seal endovascular aortic grafts is safe and effective for short-neck abdominal aortic aneurysm patients, two-year data from the ANCHOR global registry have shown.9
CX 2019: An off-the-shelf endograft (Zenith T-branch, Cook Medical) for the treatment of thoracoabdominal aortic aneurysms provides a safe and straightforward solution for a wide range of patients—including emergency cases.10
CX 2019: Late rupture after EVAR is a rare event—accounting for 15% of all ruptured aneurysms cases 10 years after the procedure. However, there has been a slow increase in rupture incidence to the extent that it had reached 28% at the 10-year point.11
CX 2019: The acute performance (30-days) results for the Valiant Navion (Medtronic) thoracic endovascular aortic repair (TEVAR) device are encouraging, with no access or deployment failures in tortuous anatomy. Furthermore, Valiant Navion has an improved design compared with current generation TEVAR devices.12
CX 2019: EVAR using proximal polymer sealing (Ovation, Endologix) does not seem to induce neck dilatation like other endografts. Moreover, in contrast to self-expanding EVAR devices, patients treated with the largest size Ovation do not suffer from more complications compared to standard diameter devices.13
CX 2019: Coil embolisation for at-risk patients during EVAR is a safe and reproducible procedure, according to a study comparing EVAR plus coil embolisation with EVAR alone. For all time points (one, six, 12 and 24 months), the number of endoleaks was lower in the cohort treated with EVAR and coils than those treated with EVAR alone. 14
CX 2019: EVAR with an iliac branch endoprothesis (Gore) is safe and effective. The procedure was associated with improved quality of life in the short term and walking capacity was preserved.15
Journal of Vascular Surgery: In a nationwide analysis of diabetic Veterans Affairs patients, prescription for metformin was associated with decreased abdominal aortic aneurysm enlargement. These findings provide further support for the conduct of prospective clinical trials to test the ability of metformin to limit progression of early disease.16
Pipeline Insights
August 2019: Ascyrus Medical has received Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections. A press release reports that this designation serves as validation of the clinical importance of the AMDS and represents a significant milestone in the continued advancement of the technology globally.17
August 2019: Endologix has announced the investigational device exemption (IDE) approval from the FDA to commence a new pivotal study to evaluate the safety and effectiveness of the Nellix Chimney EndoVascular Aneurysm Sealing System (chEVAS) for the endovascular treatment of complex abdominal aortic aneurysms.18
July 2019: Centerline Biomedical has received 510(k) clearance from the FDA to market its flagship product, the intraoperative positioning system (IOPS). A press release reports that IOPS is a non-radiation-based surgical navigation system for minimally invasive surgery that leverages anatomical mapping algorithms and electromagnetic tracking technology to provide 3D colour visualisation and guidance in real time during endovascular procedures.19
June 2019: The EC Certificate of Conformity (CE mark) for the Nellix endovascular aneurysm sealing system (Nellix system, Endologix) has been reinstated by GMED, the EU Notified Body for the Nellix system. The reinstatement followed an assessment of clinical evidence.20
May 2019: The FDA has granted regulatory approval for commercial distribution for the Gore TAG Conformable Thoracic Stent Graft with Active Control System, a thoracic endovascular aortic repair (TEVAR) solution that is designed to combine new levels of control with the performance of the conformable Gore TAG device.21
March 2019: Endospan has received the CE mark for the Nexus stent graft system to be used for branched endovascular repair in the aortic arch.22
References
- Endurant II stent graft systems
- Medtronic data expands on key aortic solutions for challenging anatomies.
- NICE abdominal aortic aneurysm guideline takes an “unprecedented” turn.
- Abdominal aortic aneurysm: diagnosis and management.
- Patterson D. Presentation at BSET 2019.
- Stenson K. Presentation at BSET 2019.
- Makaroun M. Presentation at VAM 2019.
- Katsargyris A. Presentation at Critical Issues 2019.
- Arko F. Presentation at CX 2019.
- Austerman M. Presentation at CX 2019.
- Brizzi V. Presentation at CX 2019.
- Verzini F. Presentation at CX 2019.
- Verhagen H. Presentation at CX 2019.
- D. Presentation at CX 2019.
- Verzini F. Presentation at CX 2019.
- Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg 2019; 69 (3): 710–16.
- FDA grants Breakthrough Device Designation to stent for aortic dissection
- Endologix receives FDA approval for chEVAS IDE study and signs distribution agreement with Boston Scientific.
- US FDA 510(k) clearance for intraoperative positioning system.
- Endologix announces reinstatement of CE mark for its Nellix endovascular aneurysm sealing system.
- Gore receives FDA approval for the Gore TAG conformable thoracic stent graft with Active Control.
- Endospan secures CE mark approval for branched endovascular aortic arch repair with Nexus.





