Orsiro Mission
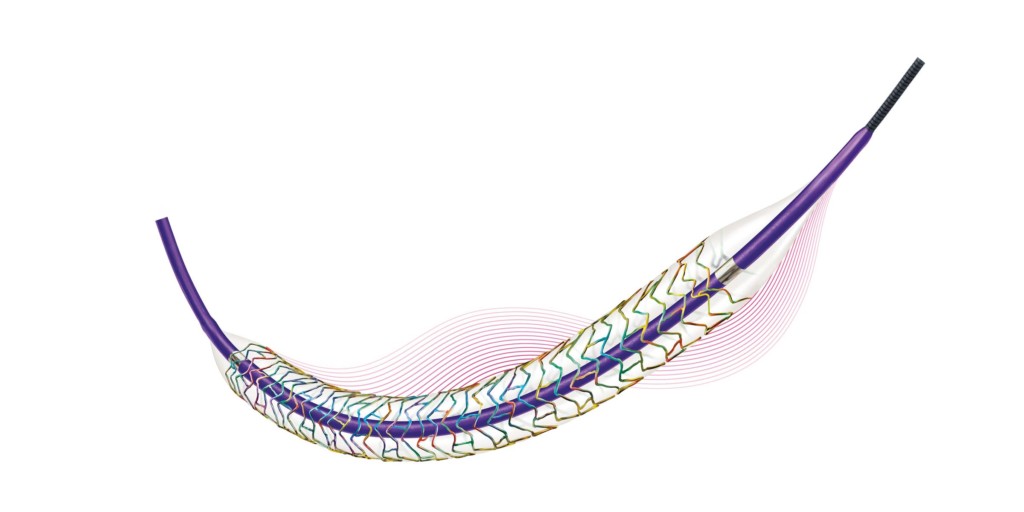
Biotronik now has the CE mark for its Orsiro Mission drug-eluting stent system. According to a press release, the next-generation of the company’s ultrathin strut Orsiro drug-eluting stent provides higher deliverability than other contemporary stents. The press release states that the Orsiro Mission “has a completely re-engineered delivery system to support interventionalists with outstanding deliverability even in challenging cases”.
The Orsiro Mission is designed to provides the benefits of the Orsiro stent. The coating combines passive and active components to avoid interaction with surrounding tissue while ensuring a controlled drug release. The stent’s ultrathin 60μm struts (≤3.0mm in diameter) aims to reduce blood flow disturbance and support early endothelialisation.
The new stent is indicated for improving coronary luminal diameter in patients with symptomatic ischaemic heart disease due to discrete de‑novo stenotic and in-stent restenotic lesions. Based on the clinical evidence with Orsiro drug-eluting stent, Orsiro Mission has 10 additional indications— including acute coronary syndrome, ST‑elevation myocardial infarction (STEMI), diabetes mellitus and complex (B2/C) lesions.
Mathias Brandt (University Hospital, Salzburg, Austria) comments: “The Orsiro Mission stent system performed smoothly under critical conditions in highly complex interventions. After having used a significant amount of this newest generation stent, this is my first choice for even the most challenging lesions.”
Alexander Uhl, president vascular Iintervention at Biotronik, states: “Orsiro Mission combines the best of two worlds—the outstanding Orsiro stent and the next level of deliverability. With more than 700 ‘real world’ evaluations successfully completed so far, Orsiro Mission is another example of how our products can make a difference in today’s patient care. By continuously improving the performance of our products, we support physicians in achieving outstanding patient outcomes.”