Source: BIBA MedTech Insights
At the American College of Cardiology/World Congress of Cardiology’s virtual scientific sessions (ACC.20/WCC Virtual; 28–30 March, Chicago, USA), D Scott Lim (University of Virginia, Charlottesville, USA) reported that mitral regurgitation ≤1+ was achieved more frequently with MitraClipNTR or with MitraClipXTR (both Abbott) than previously observed with the first-generation MitraClip device. He added that MitraClipXTR was associated with greater reduction in mitral regurgitation than was MitraClipNTR in patients with more complex anatomies.
Lim said that no core-lab adjudicated data on patients with primary mitral regurgitation had been reported since the EVEREST II trials, which evaluated the first-generation MitraClip device. He added that, over the last four years, four new generations of MitraClip have come onto the market: MitraClipNTR (2016), MitraClipNTX (2018), MitraClipXTR (2018), and MitraClipG4 (2019).
Therefore, Lim commented, the primary objective of the present study, was to report on “real-world, echo core lab and clinical events committee adjudicated outcomes in patients with significant primary mitral regurgitation treated with next-generation NTR and XTR systems in the 1,000 plus patient global EXPAND study”. Lim added that, compared with the first-generation device, MitraNTR has an improved delivery system while MitraClipXTR had “longer arms with an improved delivery catheter system”.
In the study, Lim et al reviewed data for 422 patients with primary or mixed mitral regurgitation aetiology. Of patients with primary mitral regurgitation, 46.2% received the MitraClipXTR device, 34.8% received MitraClipNTR, and 19% received both—meaning the XTR device was more frequently used to treat primary mitral regurgitation than was the NTR device. Choice of device was at the operator’s discretion but Lim commented: “Early on, a group of core members of the study got together and issued their recommendations. So for patients with greater flail whipping and for those with longer leaflets, it was advised to use the longer clip arms [MitraClip XTR]. Conversely for those with more restricted leaflets, the shorter arms of the NTR system was seen as more beneficial; it was also seen as more beneficial for patients with functional aetiologies, particularly those with more restrictive pathologies. Lastly, for patients with smaller mitral orifice areas, we thought the NRT would be superior.”
Furthermore, of those who received a MitraClipXTR device, 73.7% had severe mitral regurgitation (≥3+) vs. 26.3% who had moderate mitral regurgitation (≤2+). By comparison, of those who only received an NTR device, 47.6% had moderate disease vs. 52.4% who had severe disease.
Overall, according to echo core lab adjudicated analysis, mitral regurgitation reduction to none/trace was achieved in 27.7% of patients, reduction to ≤1+ was achieved in 86.9%, and reduction to ≤2+ was achieved in 97.3% of patients at 30 days. Lim noted: “Mitral regurgitation reduction to ≤1+ at 30 days was numerically greater in EXPAND compared to the prohibitive risk cohort from prior EVEREST studies [53.6%].” Furthermore, after comparing outcomes of the MitraClipXTR with those of the MitraClipNTR, he reported: “Greater mitral regurgitation was achieved using the MitraClipXTR in complex anatomies.”
“The study represents the first contemporary report of echo core lab and clinical events committee adjudicated 30-day clinical outcomes in patients with primary mitral regurgitation treated with next-generation NTR and XTR MitraClip devices. Approximately one third of patients had a complex mitral valve anatomy that reflects the difference in patients treated in the real world in comparison to past clinical trials,” Lim stated.
Summing up the results, he said: “The EXPAND study confirms the safety and effectiveness of the next-generation MitraClipNTR/XTR system in primary mitral regurgitation contemporary, real-world setting.”
Use of MitraClip in clinical practice
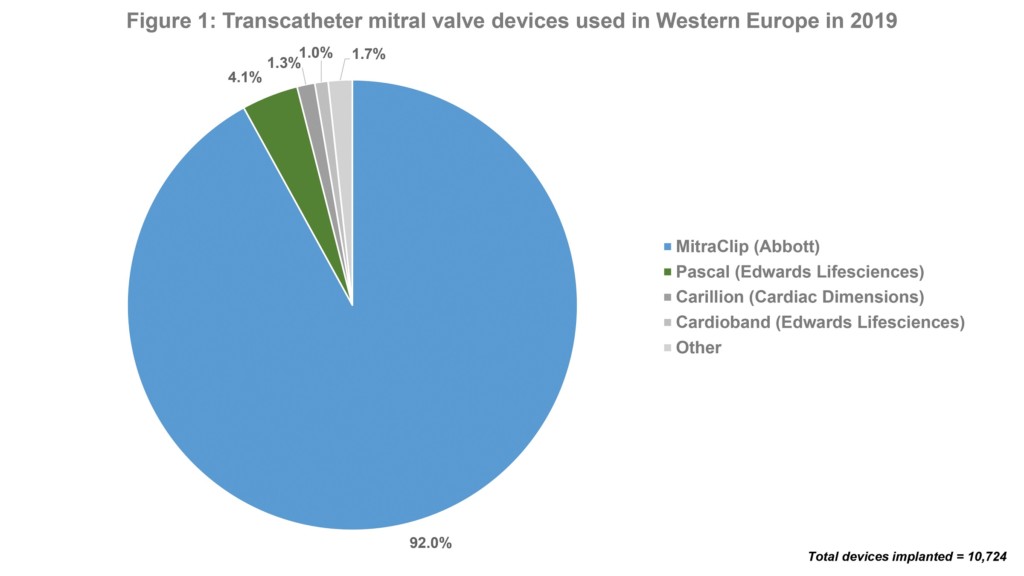
MitraClip is, by far, the most commonly used device for transcatheter mitral valve repair in Europe. Data from the BIBA MedTech Insights’ Mitral and Tricuspid Monitor shows that out of 10,724 transcatheter mitral devices used in Europe in 2019, 92% were a MitraClip device. This is to be expected as MitraClip is the most well-known device and has the most clinical data. Of note, Pascal (Edwards Lifesciences)—which, like MitraClip, is a clip-based device—was the second most used device in Europe last year. This is despite it only receiving the CE mark in February 2019. See Figure 1.
Source: BIBA MedTech Insights
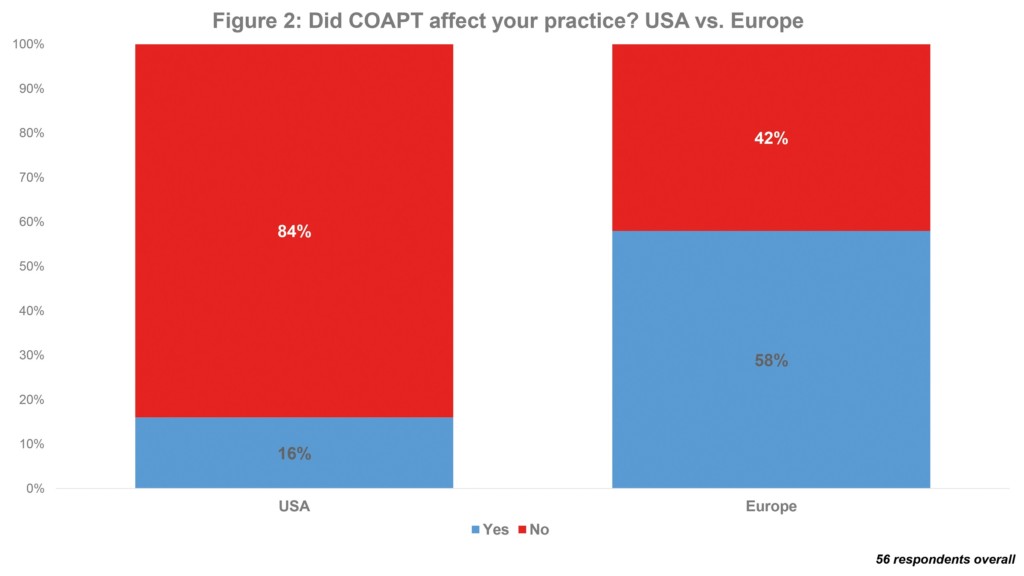
MitraClip is also most used transcatheter mitral valve device in the USA: of 5,626 implanted overall last year, 94% were a MitraClip. Again, this is to be expected; particularly as it is the only such device that has US Food and Drug Administration (FDA) approval. However, a short BIBA MedTech Insights survey, into the usage and attitudes of physicians performing transcatherter mitral valve repairs, did have a surprising finding. This indicates that the US COAPT trial appears to have had a greater impact in Europe than in the USA. The COAPT trial indicated that transcatheter mitral valve repair with MitraClip was associated with significant reductions in the rate of heart failure hospitalisations at two years for the management of secondary mitral valve regurgitation. The trial subsequently led to MitraClip receiving FDA approval for this indication (previously, it only had approval for primary mitral valve regurgitation). In Europe, MitraClip already had market approval for secondary mitral valve regurgitation. However, according to the survey, only 16% of US respondents said that the trial affected their practice vs. 58% of European physicians. See Figure 2.
The reasons for this are unclear but may be because the FDA approval, rather than the trial, had more of an impact.





