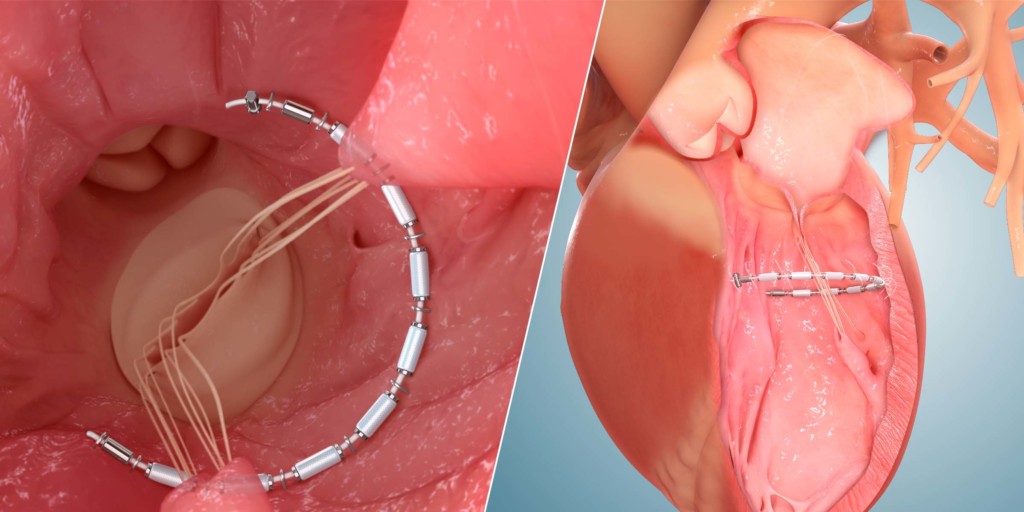
AccuCinch
The FDA has approved an expansion of enrolment of the CorCinch HFrEF early feasibility study, which is evaluating the AccuCinch ventricular repair system (Ancora Heart) for the management of patients with reduced ejection fraction heart failure (HFrEF). This expansion allows Ancora Heart to double patient enrolment from 15 to 30 patients and add an additional 10 institutions for a total of 25.
A press release reports that the transcatheter AccuCinch procedure is designed to complement and enhance the existing care cardiologists provide to further manage symptoms and slow, or stop, the progression of heart failure. It adds that for some patients, the AccuCinch procedure may have the potential to reverse the enlargement of the left ventricle. For patients whose heart failure has progressed beyond the ability of medications and pacemakers to manage symptoms, minimally invasive percutaneous device therapy with the AccuCinch system may provide an effective treatment option. system is designed to directly repair the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
Ulrich Jorde (Heart Failure, Cardiac Transplantation and Mechanical Circulatory Support, Montefiore Medical Center, New York, USA), who is the co-principal investigator of the CorCinch HFrEF study, comments: “Because heart failure is a progressive disease, medication therapy can become less effective over time, leaving patients with few treatment options as their condition worsens. The data we have gathered so far indicates that the AccuCinch system has the potential to fill this gap in treatment options which, if validated in the pivotal study, would be of great benefit to patients with heart failure.”
Jeff Closs, president and CEO of Ancora Heart, says: “The expansion of the CorCinch HFrEF study further validates the safety data we have seen in our earlier studies of the AccuCinch Ventricular Repair System in heart failure patients. By increasing the number of participating institutions and patients enrolled, we will be able to continue gaining additional clinical experience in preparation for our pivotal study.”





