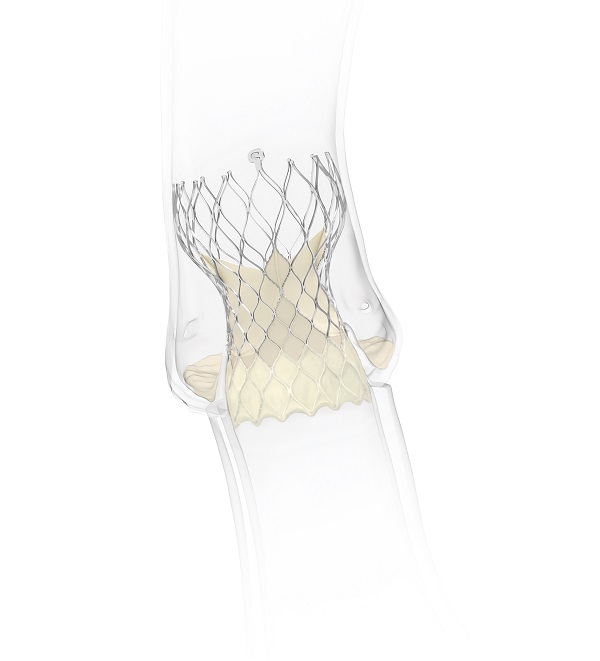
Evolut
Medtronic has been awarded CE mark for the use of the Evolut transcatheter aortic valve implantation (TAVI) system for patients with severe native aortic stenosis who are at low surgical risk. The Evolut TAVI platform has also received a new indication approval for the treatment of patients with bicuspid aortic valves who are at intermediate, high and extreme risk of surgical mortality.
With this approval, the Evolut TAVI platform is now indicated in Europe for severe aortic stenosis patients across all risk categories (extreme, high, intermediate and low), and the new labelling allows for the treatment of patients with bicuspid aortic valves who are at extreme, high and intermediate risk of surgical mortality. The Evolut TAVI platform is indicated in the USA for symptomatic severe aortic stenosis patients across all risk categories (extreme, high, intermediate and low). Bicuspid valve patients at intermediate risk or higher may be candidates for TAVI in the USA.
A press release from the company states that the expanded low-risk indication approval is based on clinical data from the global, prospective, randomised, multicentre Evolut Low Risk Trial, which evaluated three valve generations (CoreValve, Evolut R and Evolut PRO) against surgical aortic valve replacement in more than 1,400 patients. The data showed TAVI to have an excellent safety profile and be an effective treatment option in low-risk patients with shorter hospitals stays and improved 30-day quality-of-life scores compared to surgery. In addition to a lower rate of the composite of all-cause death or disabling stroke with TAVI at 30 days, Medtronic says the Evolut system demonstrated superior haemodynamic (blood flow) performance with significantly lower mean aortic valve gradients and larger EOAs (effective orifice area) compared to surgery at one year—factors that may be important for more active patients. The rate of new pacemaker implantation and residual aortic regurgitation was higher in the TAVI group.
“It is important that we expand access to a less invasive treatment option to this low-risk patient population. It’s also encouraging that we now have new labelling to address a large portion of bicuspid valve patients, too,” says Didier Tchétché (Clinique Pasteur, Toulouse, France), investigator in the Evolut Low Risk Trial. “Based on excellent data from the STS/ACC TVT Registry, bicuspid patients (excluding low risk), will for the first time, be indicated for TAVI, which is another big win for patients and the future of the therapy.”
Bicuspid aortic valves are a congenital heart defect affecting 1–2% of the general population, and is an abnormality of the aortic valve resulting in the patient having two functional valve leaflets instead of the more common three leaflets (tricuspid). Bicuspid aortic valve stenosis represents almost 40% of the intermediate and high risk severe symptomatic aortic stenosis patient population, according to the Medtronic statement.
Pieter Kappetein, vice president and chief medical officer for the Structural Heart and Cardiac Surgery businesses, part of the Cardiac and Vascular Group at Medtronic, says: “With these approvals, more patients will now be candidates for the Evolut TAVI system, while surgical aortic valve replacement will evolve to serve a more complex patient population. Medtronic is well positioned to provide a variety of therapy options to meet the varying needs of patients with heart valve disease.”





