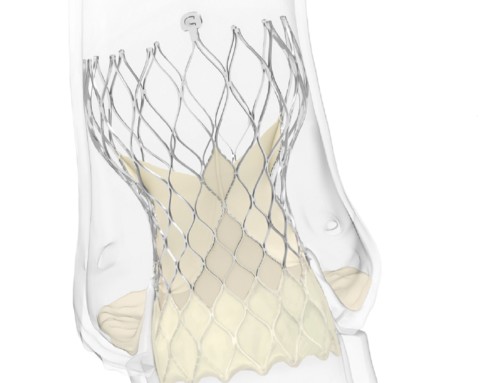
TriClip
Abbott has received the CE mark for its TriClip transcatheter tricuspid valve repair system and is now approved for use in Europe and other countries that recognise the CE mark. It can, therefore, be used as a non-surgical treatment for people with a leaky tricuspid valve. A press release reports that TriClip leverages the same clip-based technology as MitraClip, which is CE marked for transcatheter mitral valve repair, but has a differentiated delivery system designed specifically for delivery to the tricuspid valve.
The TriClip procedure repairs the tricuspid valve without the need for open-heart surgery. The device is delivered to the heart through the femoral vein in the leg and works by clipping together a portion of the leaflets of the tricuspid valve to reduce the backflow of blood. This approach is designed to allow the heart to pump blood more efficiently, relieving symptoms of tricuspid and improving a person’s quality of life.
According to a press release, the CE mark for TriClip follows positive six-month data from Abbott’s pivotal TRILUMINATE study examining edge-to-edge repair technique using TriClip (published in The Lancet in November 2019). The study demonstrated that TriClip reduced severity of tricuspid regurgitation and was associated with strong improvement in functional capacity and in quality of life at six months.
With the CE mark designation, Abbott’s TriClip device is the first minimally invasive, clip-based tricuspid valve repair device to be commercially available in the world. Abbott has now brought to market three therapies for structural heart disease: MitraClip for mitral valve repair, Tendyne for mitral valve replacement, and now TriClip to treat the tricuspid valve.
Georg Nickenig (Department of Cardiology, University Hospital, Bonn, Germany), lead investigator of the TRILUMINATE trial, says: “Patients suffering from severe tricuspid regurgitation are extremely ill and have very few treatment options. Abbott’s TriClip could profoundly impact how physicians treat these patients. The therapy is backed by data proving safety and performance, durability, and improved patient quality of life.”
Michael Dale, senior vice president of Abbott’s structural heart business, comments: “Tricuspid regurgitation is a highly prevalent, yet seldom treated disease, which is why this approval is a significant milestone for the healthcare community. TriClip has the potential to fill a treatment gap and transform how doctors are able to help people with tricuspid regurgitation. Our clip-based technology provides clinicians a life-changing, proven safe, simple, and effective option to treat people suffering from a crippling and life-threatening disease.”